44 which identifier is used to mark the label of a controlled substance?
Pharmacology Study Guide Flashcards Which identifier is used to mark the label of a controlled substance? "C" followed by a roman numeral. 21 C.f.r. § 1306.14 (a) The pharmacist filling a written or emergency oral prescription for a controlled substance listed in Schedule II shall affix to the package a label showing date of filling, the pharmacy name and address, the serial number of the prescription, the name of the patient, the name of the prescribing practitioner, and directions for use and …
Drug Safety Flashcards Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral.

Which identifier is used to mark the label of a controlled substance?
Medical Assisting Final Flashcards Which identifier is used to mark the label of a controlled substance? "CS" followed by the DEA registration number "CS" followed by a Roman numeral Medical Pharmacology Part Two AES Flashcards Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral. With whom must a medical provider register in order ... Drug Safety Quiz Flashcards Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral What is the best way to dispose of an expired controlled substance? By using a DEA-approved disposal company Which of the following is the best way to dispose of expired medications? Give expired medications to patients.
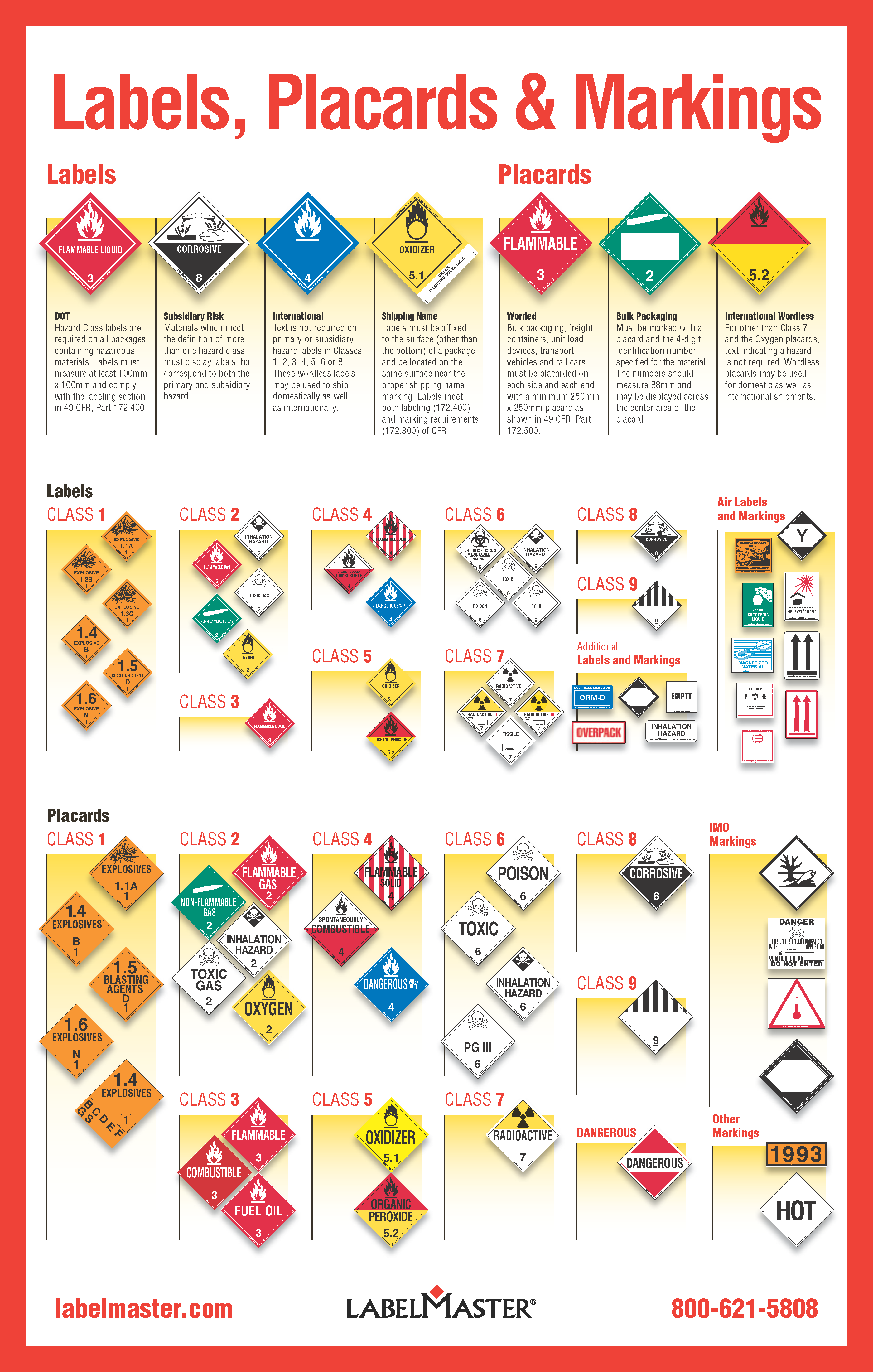
Which identifier is used to mark the label of a controlled substance?. Logical Observation Identifiers Names and Codes (LOINC) for Human ... 34085-1 CONTROLLED SUBSTANCE SECTION 9.1 Controlled Substance subsection 34086-9 ABUSE SECTION 9.2 Abuse subsection 34087-7 DEPENDENCE SECTION 9.3 Dependence subsection Quality System Regulation Labeling Requirements | FDA Various sections of the QS regulation have an impact on labeling: Section 21 CFR 820.80 (b) requires the inspection and testing of incoming materials including labeling; and 21 CFR 820.70 (f)... eCFR :: 21 CFR 1306.14 -- Labeling of substances and filling of ... (a) The pharmacist filling a written or emergency oral prescription for a controlled substance listed in Schedule II shall affix to the package a label showing date of filling, the pharmacy name and address, the serial number of the prescription, the name of the patient, the name of the prescribing practitioner, and directions for use and cautionary ... PDF DOT CHART 16 Hazardous Materials Markings,Labeling and Placarding Guide The ORGANIC PEROXIDE label [§172.427] indicates that organic peroxidesare highly flammable. Use of the ORGANIC PEROXIDE label eliminates theneed for a flammable liquid subsidiary label. The color of the border must beblack and the color of the flame may be black or white.
Controlled Substances: Determine if a Chemical is a Controlled Substance Listed chemicals (also known as precursor chemicals) Chemicals that may be used to manufacture controlled substances. Listed chemicals are regulated in order to prevent the illicit manufacture of CS. Questions? Contact the Controlled Substances Program, (858) 534-1362 or (858) 534-9016. SPL 101 “THE BASICS” States of America and other countries Used by permission All rights. States of America and other ... The Structured Product Labeling (SPL) is a document.117 pages Medical Assisting Pharmacology Test Flashcards Any substance that alters the function in a living organism. An immunization is an example of a ____ drug. Preventative. The name Lexapro® is an example of a ____. ... Most oral medications use a ____ action, which results in medication being carried through the body by the bloodstream. systemic. What is the therapeutic effect of a drug? What Is a Controlled Substance? (DEA Drug Classifications) The controlled substances act is a legal framework that defines federal drug policy in the United States. It governs the manufacture, importation, possession, and distribution of controlled substances. The purpose of the controlled substances act is to enhance controlled substance regulation.
CCOHS: WHMIS - Labels Signal word – a word used to alert the reader to a potential hazard and to indicate the severity of the hazard. Hazard statement(s) – standardized phrases which ... Implementation Guidelines for Alcohol and Drug Regulations - Chapter 6 The FMCSA regulation requires testing for the following controlled substances (or their metabolites): marijuana, cocaine, opiates, phencyclidine (PCP), and amphetamines. The regulatory requirement is found in 49 CFR, section 40.85. You may test for additional controlled substances under your own authority provided that 1. Pharmacist's Manual - DEA Diversion Jul 20, 2022 — 4. The date on which the transfer of controlled substances will occur. Page 18. Drug Enforcement Administration.124 pages CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a) Each commercial container of a controlled substance (except for a controlled substance excepted by the Administrator pursuant to § 1308.31 of this chapter) shall have printed on the...
What Information Should Be on Drug Labels? The Pr symbol indicates that the item is a prescription drug. No Pr denotes that no prescription is required to purchase it. This does not necessarily imply that it is available for purchase over the counter. Drug identification number Before any drug can be legally sold, it must be assigned a Drug Identification Number (DIN).
PDF Menu of State Prescription Drug Identification Laws controlled substance . . ."15 Similarly, an Oklahoma regulation requires identification when the pharmacist is "unsure" of the identity of the person picking up the prescription.16 An Idaho law reverses the standard, making an exception to the identification requirement "if the individual receiving the controlled substance is personally
Medical Assistant Pharmacology : Drug Safety Flashcards Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral. 3 MULTIPLE CHOICE OPTIONS.
21 CFR Part 1302 -- Labeling and Packaging ... ( a) Each commercial container of a controlled substance (except for a controlled substance excepted by the Administrator pursuant to § 1308.31 of this chapter) shall have printed on the label the symbol designating the schedule in which such controlled substance is listed.
Drug Safety Quiz Flashcards Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral What is the best way to dispose of an expired controlled substance? By using a DEA-approved disposal company Which of the following is the best way to dispose of expired medications? Give expired medications to patients.
Medical Pharmacology Part Two AES Flashcards Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral. With whom must a medical provider register in order ...
Medical Assisting Final Flashcards Which identifier is used to mark the label of a controlled substance? "CS" followed by the DEA registration number "CS" followed by a Roman numeral






























Komentar
Posting Komentar